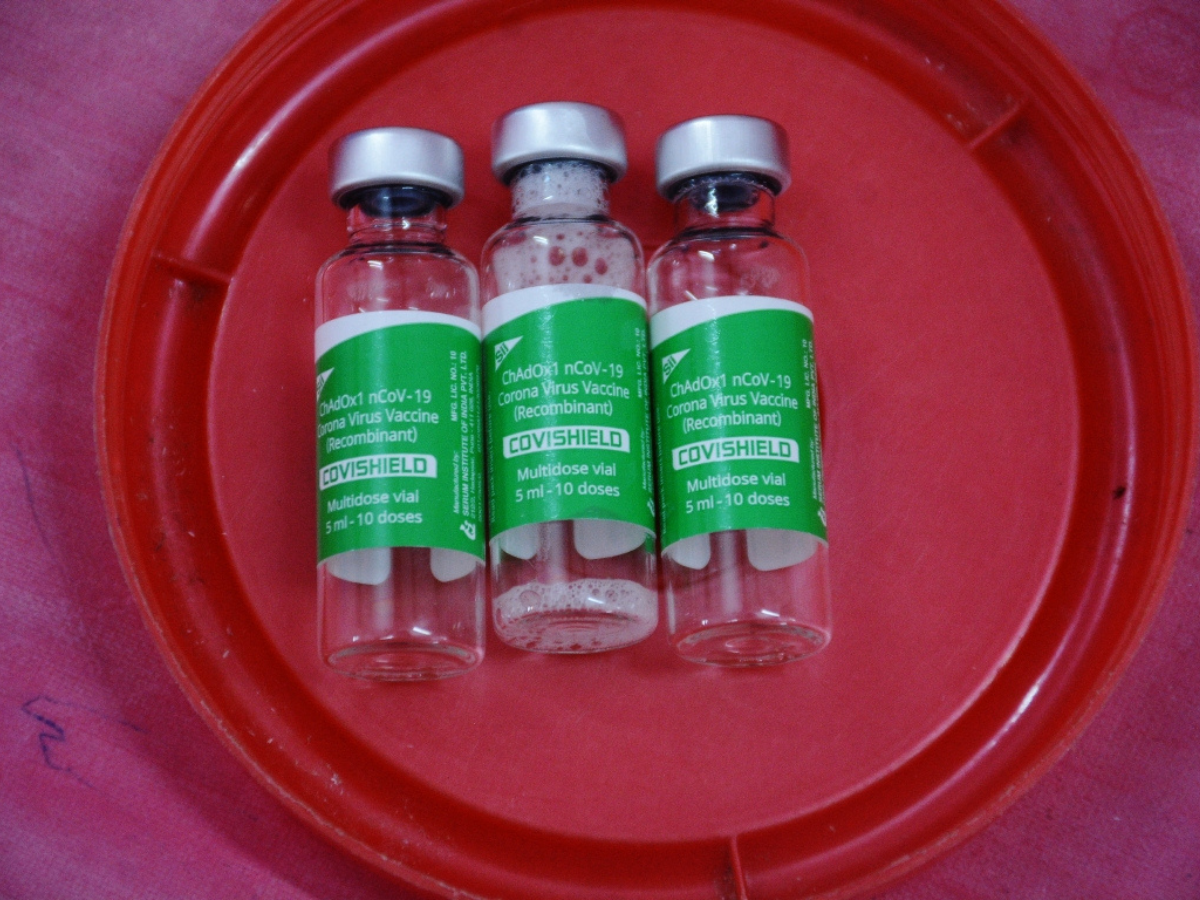
Amid the Covishield row, the medical experts on Thursday stated that the risk of Thrombosis with Thrombocytopenia Syndrome (TTS), a rare side effect linked to blood clotting is minimal.
According to Dr Rishiraj Sinha, All India General Secretary of the Federation of All India Medical Association (FAIMA), every vaccine carries a risk of side effects and none can guarantee 100% efficacy.
Drawing a comparison, Dr Sinha said that even the widely administered birth vaccination exhibits approximately 80% accuracy, yet remains effective in preventing diseases.
He asserted that the occurrence of side effects depends on an individual’s immune response.
Other experts, who did not wish to be named, said that adverse effects associated with vaccines typically manifest within one to six weeks post-administration. Therefore, individuals in India who received the vaccine two years ago need not be concerned.
They stated that in India, the occurrence of TTS following vaccination is not well-documented.
“Only isolated cases have been reported. Hence, TTP is exceedingly rare following Covid vaccination, given the millions of vaccine doses administered,” the medical experts informed.
Although Covid vaccines may slightly elevate the risk of blood clotting, the likelihood is minimal.
“Numerous scientific studies from India and other countries have substantiated this. It’s worth noting that Covid infection significantly heightens the risk of blood clot formation, far more than Covid vaccines,” they noted.
The experts also underlined that Covid-19 itself is a known catalyst for clots, heart attacks, and strokes post-infection, and vaccinated individuals are at a reduced risk of experiencing these complications.
On April 29, the British-Swedish multinational pharmaceutical company, AstraZeneca, publicly acknowledged for the first time in court documents in the United Kingdom that the Covid-19 vaccine manufactured by them, known as Covishield in India, carries the potential risk of causing Thrombosis with Thrombocytopenia Syndrome (TTS), a rare side effect linked to blood clotting.
Covishield, produced in collaboration with the University of Oxford, was widely distributed in India by the Pune-based Serum Institute of India (SII) and played a pivotal role in mitigating severe COVID-19 complications in the country.
It is worth noting that the SII has not yet issued any official statement following the reports.
The vaccine garnered significant usage, with a staggering 1,749,417,978 doses administered in India alone. This marked a milestone as India conducted one of the largest vaccination drives globally, commencing in January 2021 and extending through April 29, 2024, as per data from the government’s CoWIN vaccine dashboard.
Responding to a recent lawsuit, AstraZeneca has expressed empathy for people affected by adverse reactions to its vaccine, saying that the company is committed to patient safety.
A study published in a medical journal last year shed light on short-term adverse effects following two doses of Covishield. However, it said that these effects are mild and transient.
Facing a class action lawsuit in the UK, AstraZeneca stands accused of causing fatalities and severe injuries in numerous cases. Victims involved in up to 51 cases in the UK High Court are seeking damages totalling up to 100 million pounds.